How To Repair Damaged Dna
DNA, like whatever other molecule, can undergo a variety of chemical reactions. Because DNA uniquely serves as a permanent copy of the cell genome, yet, changes in its structure are of much greater result than are alterations in other cell components, such as RNAs or proteins. Mutations tin can outcome from the incorporation of incorrect bases during DNA replication. In add-on, various chemical changes occur in Dna either spontaneously (Figure 5.19) or as a result of exposure to chemicals or radiation (Figure 5.20). Such damage to Deoxyribonucleic acid can block replication or transcription, and can result in a high frequency of mutations—consequences that are unacceptable from the standpoint of cell reproduction. To maintain the integrity of their genomes, cells accept therefore had to evolve mechanisms to repair damaged Dna. These mechanisms of DNA repair can exist divided into ii general classes: (1) direct reversal of the chemical reaction responsible for DNA harm, and (2) removal of the damaged bases followed by their replacement with newly synthesized DNA. Where Deoxyribonucleic acid repair fails, boosted mechanisms have evolved to enable cells to cope with the impairment.
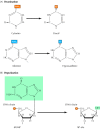
Effigy 5.nineteen
Spontaneous damage to DNA. In that location are two major forms of spontaneous DNA impairment: (A) deamination of adenine, cytosine, and guanine, and (B) depurination (loss of purine bases) resulting from cleavage of the bond betwixt the purine bases and deoxyribose, (more than...)
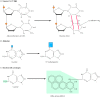
Figure v.20
Examples of Dna harm induced by radiation and chemicals. (A) UV low-cal induces the formation of pyrimidine dimers, in which two next pyrimidines (e.g., thymines) are joined by a cyclobutane ring structure. (B) Alkylation is the add-on of methyl (more than...)
Directly Reversal of Dna Harm
Virtually damage to DNA is repaired by removal of the damaged bases followed by resynthesis of the excised region. Some lesions in DNA, still, tin can exist repaired by direct reversal of the harm, which may be a more efficient style of dealing with specific types of Dna impairment that occur frequently. Only a few types of DNA damage are repaired in this way, especially pyrimidine dimers resulting from exposure to ultraviolet (UV) light and alkylated guanine residues that have been modified past the addition of methyl or ethyl groups at the O6 position of the purine ring.
UV light is ane of the major sources of damage to Dna and is also the nearly thoroughly studied form of Deoxyribonucleic acid damage in terms of repair mechanisms. Its importance is illustrated by the fact that exposure to solar UV irradiation is the crusade of most all skin cancer in humans. The major type of impairment induced by UV calorie-free is the formation of pyrimidine dimers, in which adjacent pyrimidines on the same strand of Deoxyribonucleic acid are joined by the formation of a cyclobutane ring resulting from saturation of the double bonds between carbons five and 6 (come across Figure 5.20A). The formation of such dimers distorts the structure of the DNA chain and blocks transcription or replication past the site of damage, so their repair is closely correlated with the ability of cells to survive UV irradiation. One mechanism of repairing UV-induced pyrimidine dimers is direct reversal of the dimerization reaction. The procedure is called photoreactivation because energy derived from visible light is utilized to intermission the cyclobutane band structure (Effigy 5.21). The original pyrimidine bases remain in DNA, now restored to their normal country. As might be expected from the fact that solar UV irradiation is a major source of DNA damage for various cell types, the repair of pyrimidine dimers by photoreactivation is mutual to a variety of prokaryotic and eukaryotic cells, including E. coli, yeasts, and some species of plants and animals. Curiously, even so, photoreactivation is non universal; many species (including humans) lack this machinery of Dna repair.

Figure 5.21
Direct repair of thymine dimers. UV-induced thymine dimers can be repaired past photoreactivation, in which energy from visible calorie-free is used to split the bonds forming the cyclobutane ring.
Another grade of straight repair deals with damage resulting from the reaction between alkylating agents and DNA. Alkylating agents are reactive compounds that can transfer methyl or ethyl groups to a Deoxyribonucleic acid base, thereby chemically modifying the base (come across Figure 5.20B). A particularly important type of damage is methylation of the O6 position of guanine, because the product, O6-methylguanine, forms complementary base pairs with thymine instead of cytosine. This lesion can exist repaired by an enzyme (called O6-methylguanine methyltransferase) that transfers the methyl group from O6-methylguanine to a cysteine residue in its agile site (Figure v.22). The potentially mutagenic chemical modification is thus removed, and the original guanine is restored. Enzymes that catalyze this direct repair reaction are widespread in both prokaryotes and eukaryotes, including humans.

Figure v.22
Repair of O 6 -methylguanine. O6-methylguanine methyltransferase transfers the methyl group from O6-methylguanine to a cysteine residue in the enzyme'southward active site.
Excision Repair
Although direct repair is an efficient way of dealing with particular types of DNA impairment, excision repair is a more general means of repairing a wide variety of chemical alterations to DNA. Consequently, the diverse types of excision repair are the most important DNA repair mechanisms in both prokaryotic and eukaryotic cells. In excision repair, the damaged DNA is recognized and removed, either equally free bases or as nucleotides. The resulting gap is then filled in by synthesis of a new DNA strand, using the undamaged complementary strand as a template. Three types of excision repair—base of operations-excision repair, nucleotide-excision repair, and mismatch repair—enable cells to cope with a diversity of different kinds of DNA harm.
The repair of uracil-containing DNA is a skillful example of base-excision repair, in which single damaged bases are recognized and removed from the DNA molecule (Effigy 5.23). Uracil tin can arise in DNA by two mechanisms: (1) Uracil (as dUTP [deoxyuridine triphosphate]) is occasionally incorporated in place of thymine during Deoxyribonucleic acid synthesis, and (2) uracil can be formed in DNA by the deamination of cytosine (see Figure v.19A). The second mechanism is of much greater biological significance considering it alters the normal design of complementary base pairing and thus represents a mutagenic event. The excision of uracil in DNA is catalyzed by DNA glycosylase, an enzyme that cleaves the bond linking the base of operations (uracil) to the deoxyribose of the DNA courage. This reaction yields complimentary uracil and an apyrimidinic site—a sugar with no base of operations attached. Deoxyribonucleic acid glycosylases also recognize and remove other abnormal bases, including hypoxanthine formed by the deamination of adenine, pyrimidine dimers, alkylated purines other than Ohalf-dozen-alkylguanine, and bases damaged by oxidation or ionizing radiations.

Figure v.23
Base of operations-excision repair. In this instance, uracil (U) has been formed by deamination of cytosine (C) and is therefore opposite a guanine (M) in the complementary strand of Deoxyribonucleic acid. The bond between uracil and the deoxyribose is cleaved past a Dna glycosylase, leaving (more than...)
The result of DNA glycosylase action is the germination of an apyridiminic or apurinic site (more often than not chosen an AP site) in Dna. Similar AP sites are formed every bit the result of the spontaneous loss of purine bases (meet Effigy 5.19B), which occurs at a pregnant rate nether normal cellular conditions. For example, each jail cell in the human body is estimated to lose several thousand purine bases daily. These sites are repaired by AP endonuclease, which cleaves adjacent to the AP site (see Figure 5.23). The remaining deoxyribose moiety is then removed, and the resulting single-base of operations gap is filled past Deoxyribonucleic acid polymerase and ligase.
Whereas Deoxyribonucleic acid glycosylases recognize only specific forms of damaged bases, other excision repair systems recognize a wide variety of damaged bases that misconstrue the DNA molecule, including UV-induced pyrimidine dimers and beefy groups added to Deoxyribonucleic acid bases equally a outcome of the reaction of many carcinogens with Deoxyribonucleic acid (see Figure v.20C). This widespread course of Dna repair is known as nucleotide-excision repair, considering the damaged bases (e.g., a thymine dimer) are removed as function of an oligonucleotide containing the lesion (Figure five.24).
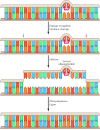
Figure 5.24
Nucleotide-excision repair of thymine dimers. Damaged DNA is recognized and so cleaved on both sides of a thymine dimer past 3′ and v′ nucleases. Unwinding by a helicase results in excision of an oligonucleotide containing the damaged (more...)
In E. coli, nucleotide-excision repair is catalyzed by the products of three genes (uvrA, B, and C) that were identified considering mutations at these loci event in farthermost sensitivity to UV low-cal. The poly peptide UvrA recognizes damaged Deoxyribonucleic acid and recruits UvrB and UvrC to the site of the lesion. UvrB and UvrC then cleave on the iii′ and five′ sides of the damaged site, respectively, thus excising an oligonucleotide consisting of 12 or 13 bases. The UvrABC circuitous is frequently chosen an excinuclease, a proper name that reflects its ability to directly excise an oligonucleotide. The action of a helicase is then required to remove the damage-containing oligonucleotide from the double-stranded Deoxyribonucleic acid molecule, and the resulting gap is filled past DNA polymerase I and sealed by ligase.
Nucleotide-excision repair systems have also been studied extensively in eukaryotes, particularly in yeasts and in humans. In yeasts, as in E. coli, several genes involved in DNA repair (called RAD genes for radiation sensitivity) have been identified past the isolation of mutants with increased sensitivity to UV light. In humans, DNA repair genes take been identified largely by studies of individuals suffering from inherited diseases resulting from deficiencies in the ability to repair DNA harm. The nigh extensively studied of these diseases is xeroderma pigmentosum (XP), a rare genetic disorder that affects approximately ane in 250,000 people. Individuals with this disease are extremely sensitive to UV low-cal and develop multiple skin cancers on the regions of their bodies that are exposed to sunlight. In 1968 James Cleaver made the key discovery that cultured cells from XP patients were deficient in the ability to carry out nucleotide-excision repair. This ascertainment not only provided the get-go link between DNA repair and cancer, but also suggested the employ of XP cells equally an experimental system to identify homo Dna repair genes. The identification of homo Deoxyribonucleic acid repair genes has been accomplished by studies not only of XP cells, but also of two other human being diseases resulting from Deoxyribonucleic acid repair defects (Cockayne's syndrome and trichothiodystrophy) and of UV-sensitive mutants of rodent cell lines. The availability of mammalian cells with defects in DNA repair has allowed the cloning of repair genes based on the ability of wild-type alleles to restore normal UV sensitivity to mutant cells in cistron transfer assays, thereby opening the door to experimental analysis of nucleotide-excision repair in mammalian cells.
Molecular cloning has at present identified vii different repair genes (designated XPA through XPG) that are mutated in cases of xeroderma pigmentosum, too every bit in some cases of Cockayne'due south syndrome, trichothiodystrophy, and UV-sensitive mutants of rodent cells. Table 5.one lists the enzymes encoded past these genes. Some UV-sensitive rodent cells have mutations in however some other repair factor, chosen ERCC1 (for excision repair cross complementing), which has not been found to exist mutated in known man diseases. It is notable that the proteins encoded by these human DNA repair genes are closely related to proteins encoded by yeast RAD genes, indicating that nucleotide-excision repair is highly conserved throughout eukaryotes.
Table 5.1
Enzymes Involved in Nucleotide-Excision Repair.
With cloned yeast and human repair genes bachelor, it has been possible to purify their encoded proteins and develop in vitro systems to study the repair process. Although some steps remain to be fully elucidated, these studies take led to the development of a basic model for nucleotide-excision repair in eukaryotic cells. In mammalian cells, the XPA protein (and possibly also XPC) initiates repair by recognizing damaged Dna and forming complexes with other proteins involved in the repair process. These include the XPB and XPD proteins, which act every bit helicases that unwind the damaged DNA. In improver, the binding of XPA to damaged DNA leads to the recruitment of XPF (as a heterodimer with ERCC1) and XPG to the repair complex. XPF/ERCC1 and XPG are endonucleases, which carve DNA on the v′ and 3′ sides of the damaged site, respectively. This cleavage excises an oligonucleotide consisting of approximately thirty bases. The resulting gap and then appears to be filled in past DNA polymerase δ or ε (in clan with RFC and PCNA) and sealed past ligase.
An intriguing feature of nucleotide-excision repair is its human relationship to transcription. A connexion between transcription and repair was kickoff suggested by experiments showing that transcribed strands of DNA are repaired more than rapidly than nontranscribed strands in both E. coli and mammalian cells. Since DNA damage blocks transcription, this transcription-repair coupling is thought to be advantageous by allowing the jail cell to preferentially repair harm to actively expressed genes. In E. coli, the mechanism of transcription-repair coupling involves recognition of RNA polymerase stalled at a lesion in the DNA strand existence transcribed. The stalled RNA polymerase is recognized by a poly peptide called transcription-repair coupling gene, which displaces RNA polymerase and recruits the UvrABC excinuclease to the site of damage.
Although the molecular mechanism of transcription-repair coupling in mammalian cells is not even so known, it is noteworthy that the XPB and XPD helicases are components of a multisubunit transcription factor (called TFIIH) that is required to initiate the transcription of eukaryotic genes (see Affiliate 6). Thus, these helicases appear to be required for the unwinding of DNA during both transcription and nucleotide-excision repair, providing a direct biochemical link between these two processes. Patients suffering from Cockayne's syndrome are also characterized from a failure to preferentially repair transcribed DNA strands, suggesting that the proteins encoded by the two genes known to exist responsible for this affliction (CSA and CSB) function in transcription-coupled repair. In addition, one of the genes responsible for inherited breast cancer in humans (BRCA1) appears to encode a protein specifically involved in transcription-coupled repair of oxidative DNA damage, suggesting that defects in this type of Deoxyribonucleic acid repair can lead to the evolution of i of the most common cancers in women.
A third excision repair arrangement recognizes mismatched bases that are incorporated during Dna replication. Many such mismatched bases are removed past the proofreading activeness of Deoxyribonucleic acid polymerase. The ones that are missed are discipline to later correction past the mismatch repair system, which scans newly replicated Deoxyribonucleic acid. If a mismatch is constitute, the enzymes of this repair system are able to identify and excise the mismatched base specifically from the newly replicated DNA strand, allowing the error to be corrected and the original sequence restored.
In Due east. coli, the ability of the mismatch repair system to distinguish betwixt parental Deoxyribonucleic acid and newly synthesized DNA is based on the fact that Deoxyribonucleic acid of this bacterium is modified by the methylation of adenine residues within the sequence GATC to form vi-methyladenine (Figure five.25). Since methylation occurs afterward replication, newly synthesized Dna strands are non methylated and thus can be specifically recognized by the mismatch repair enzymes. Mismatch repair is initiated by the protein MutS, which recognizes the mismatch and forms a complex with two other proteins called MutL and MutH. The MutH endonuclease then cleaves the unmethylated DNA strand at a GATC sequence. MutL and MutS then act together with an exonuclease and a helicase to excise the Dna betwixt the strand break and the mismatch, with the resulting gap being filled by DNA polymerase and ligase.

Figure v.25
Mismatch repair in East. coli. The mismatch repair organisation detects and excises mismatched bases in newly replicated DNA, which is distinguished from the parental strand because it has non still been methylated. MutS binds to the mismatched base, followed past (more than...)
Eukaryotes have a similar mismatch repair organization, although the mechanism by which eukaryotic cells identify newly replicated DNA differs from that used by E. coli. In mammalian cells, information technology appears that the strand-specificity of mismatch repair is determined by the presence of single-strand breaks (which would be present in newly replicated Dna) in the strand to be repaired (Figure v.26). The eukaryotic homologs of MutS and MutL then bind to the mismatched base and straight excision of the Deoxyribonucleic acid between the strand break and the mismatch, as in Due east. coli. The importance of this repair system is dramatically illustrated by the fact that mutations in the human being homologs of MutS and MutL are responsible for a common type of inherited colon cancer (hereditary nonpolyposis colorectal cancer, or HNPCC). HNPCC is 1 of the most mutual inherited diseases; it affects as many as one in 200 people and is responsible for about fifteen% of all colorectal cancers in this country. The relationship between HNPCC and defects in mismatch repair was discovered in 1993, when 2 groups of researchers cloned the human being homolog of MutS and establish that mutations in this gene were responsible for about half of all HNPCC cases. Subsequent studies have shown that virtually of the remaining cases of HNPCC are caused by mutations in 1 of 3 human genes that are homologs of MutL.

Figure 5.26
Mismatch repair in mammalian cells. Mismatch repair in mammalian cells is like to E. coli, except that the newly replicated strand is distinguished from the parental strand considering it contains strand breaks. MutS and MutL demark to the mismatched base (more...)
Postreplication Repair
The direct reversal and excision repair systems human action to right Dna impairment earlier replication, so that replicative Deoxyribonucleic acid synthesis tin continue using an undamaged Deoxyribonucleic acid strand as a template. Should these systems neglect, still, the cell has alternative mechanisms for dealing with damaged DNA at the replication fork. Pyrimidine dimers and many other types of lesions cannot be copied past the normal activeness of DNA polymerases, so replication is blocked at the sites of such impairment. Downstream of the damaged site, still, replication can be initiated again past the synthesis of an Okazaki fragment and can go along along the damaged template strand (Figure 5.27). The result is a girl strand that has a gap opposite the site of impairment to the parental strand. 1 of two types of mechanisms may be used to repair such gaps in newly synthesized Dna: recombinational repair or error-prone repair.

Figure 5.27
Postreplication repair. The presence of a thymine dimer blocks replication, simply Deoxyribonucleic acid polymerase can featherbed the lesion and reinitiate replication at a new site downstream of the dimer. The result is a gap opposite the dimer in the newly synthesized Deoxyribonucleic acid (more than...)
Recombinational repair depends on the fact that one strand of the parental DNA was undamaged and therefore was copied during replication to yield a normal daughter molecule (see Figure v.27). The undamaged parental strand can exist used to fill the gap opposite the site of damage in the other daughter molecule by recombination between homologous DNA sequences (meet the side by side section). Because the resulting gap in the previously intact parental strand is reverse an undamaged strand, it can be filled in by DNA polymerase. Although the other parent molecule still retains the original damage (east.yard., a pyrimidine dimer), the impairment at present lies opposite a normal strand and can be dealt with afterward by excision repair. By a similar machinery, recombination with an intact DNA molecule can be used to repair double strand breaks, which are frequently introduced into Deoxyribonucleic acid by radiations and other damaging agents.
In error-prone repair, a gap opposite a site of DNA damage is filled past newly synthesized Deoxyribonucleic acid. Since the new Deoxyribonucleic acid is synthesized from a damaged template strand, this class of Deoxyribonucleic acid synthesis is very inaccurate and leads to frequent mutations. Information technology is used but in bacteria that have been subjected to potentially lethal weather condition, such as extensive UV irradiation. Such treatments induce the SOS response, which may be viewed as a mechanism for dealing with extreme environmental stress. The SOS response includes inhibition of cell partitioning and consecration of repair systems to cope with a loftier level of DNA damage. Nether these conditions, fault-prone repair mechanisms are used, presumably equally a mode of dealing with damage so extensive that cell death is the only culling.
![]()
Box
Molecular Medicine : Colon Cancer and Deoxyribonucleic acid Repair.
Source: https://www.ncbi.nlm.nih.gov/books/NBK9900/
Posted by: castellanoslittevers.blogspot.com


0 Response to "How To Repair Damaged Dna"
Post a Comment